BOYLE'S LAW LAB REPORT RECOMMENDATIONS
Verify Boyles law Experimentally 2. Also from Avogadros Law we can conclude that the pressure of a gas depends on both volume and temperature but not composition of the gas or whether the gases are all the same orshow more content.

Boyles Law Lab Report Edu T211k4 Action Research B Part Ii Studocu
How the Law of Robert Boyle Effects the Volume of a Gas When it is Revealed to A Heightened Water Pressure.

. Students will then complete a formal lab report. In this lab we compared the changes of volume with different pressures using a barometer. This experiment will allow you to explore this relation of pressure.
Doubling the pressure cuts the volume in half. That means if the volume increases then pressure decreases or if pressure increases then volume decreases. Ters nm where 1 nm 1 109 m.
Tripling the pressure reduces the volume to one-third its original value. Boyles Law Lab. Boyles Law explains the relationship between the pressure and volume of an ideal gas when the temperature and amount of gas inside a container remain constant.
In other words the pressure and volume of a gas are inversely proportional to each other as long as the temperature and the quantity of gas are kept constant. Use Bohrs equation shown below to calculate the wavelength of light emitted in the following electronic transitions. The law that shows the relationship between these factors is called the Ideal Gas Law.
N 4 n 1 iii. Connect the open end of the syringe to the pressure sensor. Open animation file 2.
By Birdie and Jordie. After the plunger has slid down total the mass on the syringe record this value on the correct row of the data tableiii. To determine the relationship between pressure exerted and volume of a gas.
Boyles Law states that pressure and volume are inversely proportional to each other. The law is the oldest gas law. Robert Boyle discovered the inverse relationship between pressure and volume in 1662.
Open the valve and pull the plunger back to set the initial volume of the air in the cylinder at 200cm3. Boyles experiment A very detailed description of the historical experiment that led to Boyles law can be found in the Part II chapter V of the Boyles book published in 1662 6 and also in the liter-ature 12. The purpose of this lab is to investigate the relationship between the volume of a container and pressure of the gas inside the container.
Say if your values for l Hh hover in the interval 2740 2767cm2 then its probably a good idea to take the vertical axis from 2700 to 2800 cm2. Students will investigate Boyles law using syringes gas pressure sensors and labquest digital data collection units. Give your answers in nanome.
Connect the pressure sensor to Channel 1 of the LabPro. PV nRT where P is the pressure of the gas in. Boyles Law is a very important gas law in chemistry and physics.
The article covers a standard laboratory method to verify the law by studying the relation between pressure and volume. To determine the relationship between pressure and volume of an ideal gas. Experiment C-30 Boyles Law Ver 344 Introduction An ideal gas can be characterized by three parameters.
There are several ways to verify the law. Quadrupling the pressure reduces the volume to one-fourth its original value. It is also known as Mariottes law or the Boyle-Mariotte law.
Be able to distinguish between random and systematic error INTRODUCTION In this experiment we will investigate the relationship between pressure and. Boyles Law Lab Purpose. Boyle used a glass tube bended in an U shape having one.
The relative errors are about 1 or less as you may remember from the Boyles experiment done during the year. Boyles law along with Charless law Gay-Lussacs law and Avogadros law forms the ideal gas law. Using Prezi Video for virtual sales presentations that convert.
Boyles law is a gas law which states that the pressure exerted by a gas of a given mass kept at a constant temperature is inversely proportional to the volume occupied by it. Calculate the pressure using the formula P 103 mass on syringe area of top of syringeiv. Will the volume of the air decrease as the pressure exerted increases due to Boyles Law.
N 6 n 1. How to schedule fewer meetings and get more done. Boyles Law Lab Report.
It relates pressure and volume of gas keeping other parameters amount of gas and temperature constant. The lesson question of the lab was what is the effect of pressure on the volume of a gas. To compare the experimental results with theoretical results.
Obtained by Boyle in the experiment that he made in 1662. Pressure volume and temperature for a certain amount of gas. He made these observations by using Mercury in a J-tube and made measurements of the volume of the trapped gas at pressures both higher and lower then normal atmospheric pressure.
APPARATUS Pressure sensor syringe and computer. The mathematical equation for Boyles law is P1 x V1 P2 X V2 Boyles law is consistent with kinetic molecular theory. To calculate the PV.
The above relation is only approximately true. If the pressure on a gas is increased then its volume will decrease because the gas molecules will be pushed closer together. The law correlates how the pressure of a gas increases with a decrease in the.
Place the correct amount of weight on the syringe to apply additional pressure to the gas. P V constant. Get exposed to using a spreadsheet for making graphs 4.
BOYLES LAW LEARNING OBJECTIVES 1. Gas law reduces to Boyles law. Modify the number of books that press down on the piston starting from 0 books and increasing by 1 each time until 19 books.
This reveals that pressure is proportional to the inverse of volume. As the pressure increases the volume decreases and vice versa. All Chemistry Classes Boyles Law Lab Name _____ Purpose.
How to get repeat customers. Boyles Law Lab Report by Stephanye Cruz. The purpose of this lab was to prove that when pressure increases volume decreases.
The trick is to have enough room to display the points and the error bars. Boyles Law Lab Report Purpose. Record the pressure of books and.
This is also known as Boyles Law which can be dated back to 1662. Boyles Law states that at constant temperature the pressure p of the gas times its volume V will remain constant. N 2 n 1 ii.
Practice taking precise measurements 3. Boyles Law states that the volume of a dry gas is inversely proportional to the pressure if the temperature remains constant. Boyles law is a famous gas law studied in physics and chemistry.
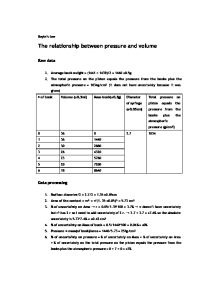
Ib Chemistry Boyle S Law Lab Report International Baccalaureate Chemistry Marked By Teachers Com
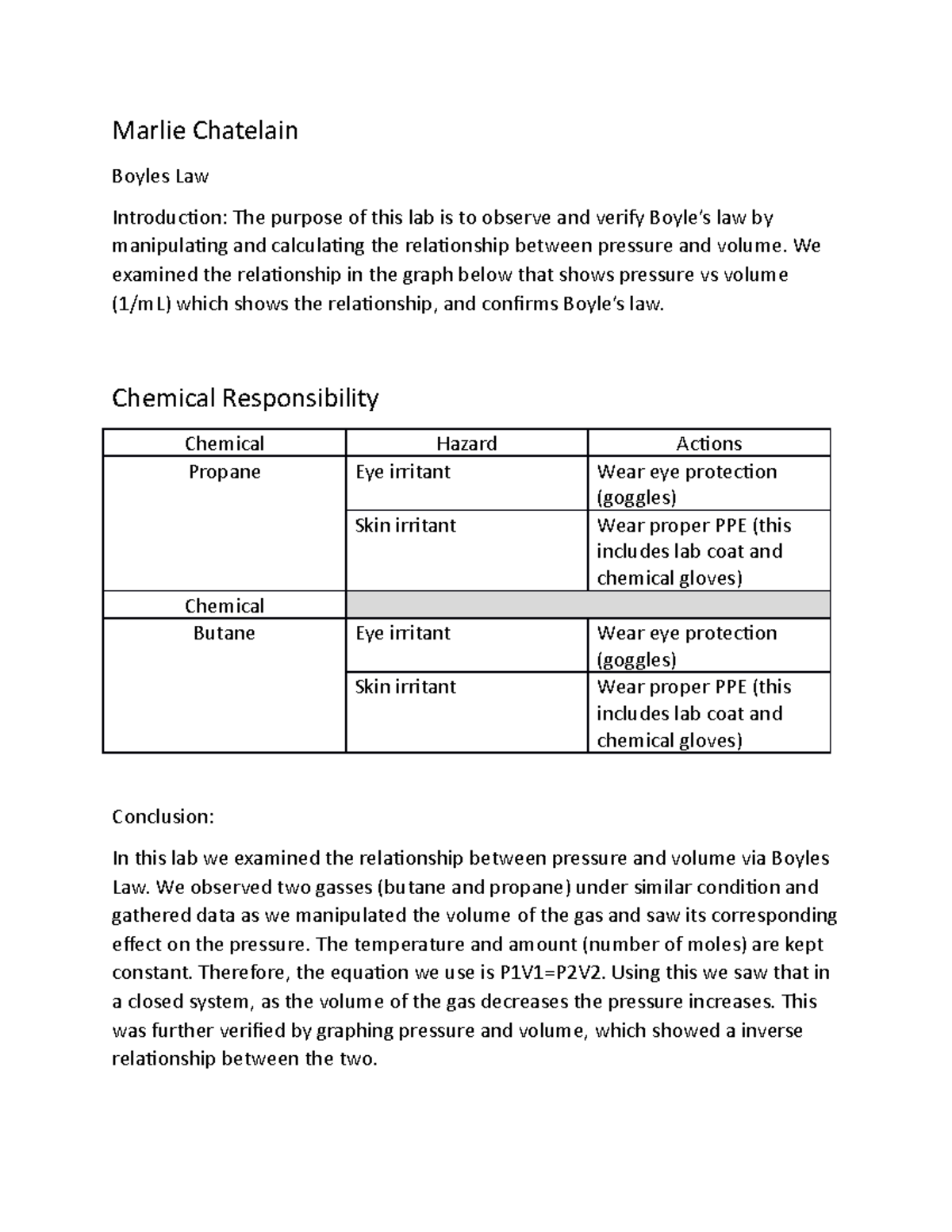
Boyle S Law Lab Chem Lab Marlie Chatelain Boyles Law Introduction The Purpose Of This Lab Is To Studocu

Ib Ia Gas Law Experiment Testing Boyles Law Charles Law And Ideal Gas Law International Baccalaureate Chemistry Marked By Teachers Com

Solved Report For Experiment 18 Instructor Boyle S Law Data Chegg Com

Solved Experiment 7 Boyle S Law Laboratory Report Show Any Chegg Com
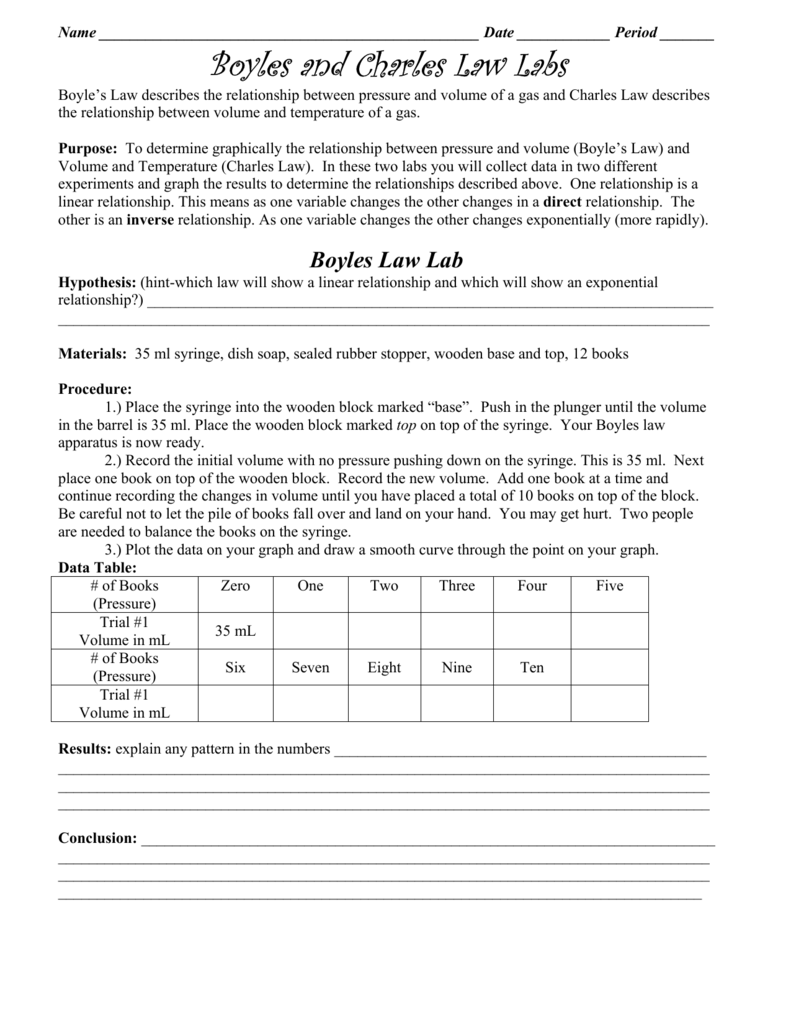
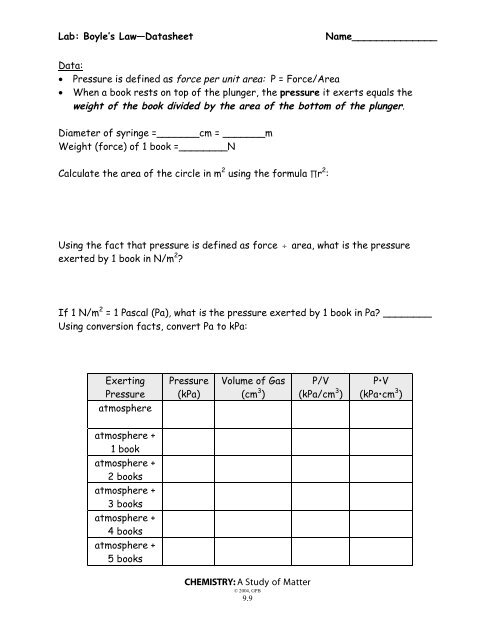
Belum ada Komentar untuk "BOYLE'S LAW LAB REPORT RECOMMENDATIONS"
Posting Komentar